
Dr. Andrea Wolf is the Director of the New York Mesothelioma Program at Mount Sinai in New York City. She focuses on multidisciplinary treatment, clinical research, community outreach and education.
Tumor Treating Fields, also known as TTFields, is a newer cancer therapy approved by the U.S. Food and Drug Administration to treat pleural mesothelioma. It works in combination with chemotherapy to limit cancer growth and improve survival.
Tumor Treating Fields for mesothelioma is a noninvasive therapy that uses electrical fields to target cancer cells. Electrical fields come from tiny charged particles like electrons in your body. These fields can push or pull on other charged particles similar to how magnets can attract or repel each other.
In mesothelioma treatment, TTFields use these electrical fields to target cancer cells without harming your healthy cells. They disrupt special proteins inside mesothelioma cells that help them divide and grow. When the fields interfere with this process, mesothelioma cells eventually die, which can help shrink your tumors over time.
Key Facts About TTFields for Mesothelioma
The U.S. Food and Drug Administration approved TTFields for pleural mesothelioma in May 2019. TTFields therapy is given along with a chemotherapy regimen of Alimta (pemetrexed) and cisplatin. Novocure has been developing TTFields since 2000.
The clinical trial that led to FDA approval reported overall survival for people with pleural mesothelioma was an average of 6 months more with TTFields than with chemo alone. TTFields therapy doesn’t offer a cure for mesothelioma, but it can extend survival with a low risk of side effects.
TTFields therapy sends gentle electrical waves through the skin to help treat mesothelioma. The electrical waves switch directions back and forth repeatedly, known as alternating electrical fields.
A 2025 study in The American Journal of Cancer Research supported previous findings that TTFields boosts chemo results. The study found TTFields therapy helps cancer cells take in more medicine and keep it inside longer.
A 2021 study in Lung Cancer also found adding TTFields to chemo increases the amount of proteins that damage cancer cells’ DNA and reduces the level of proteins that repair cancer DNA. Damaged cancer DNA leads to tumor cell death.
As Dr. Jacques Fontaine, director of Moffitt Cancer Center’s Mesothelioma Research and Treatment Center, tells us, “Basically, low-voltage electrical fields are safely applied through the skin to disrupt the electric microcurrents inside cells required for their division. It ‘jams up’ those fields inside tumor cells, not allowing them to divide, multiply or grow.”
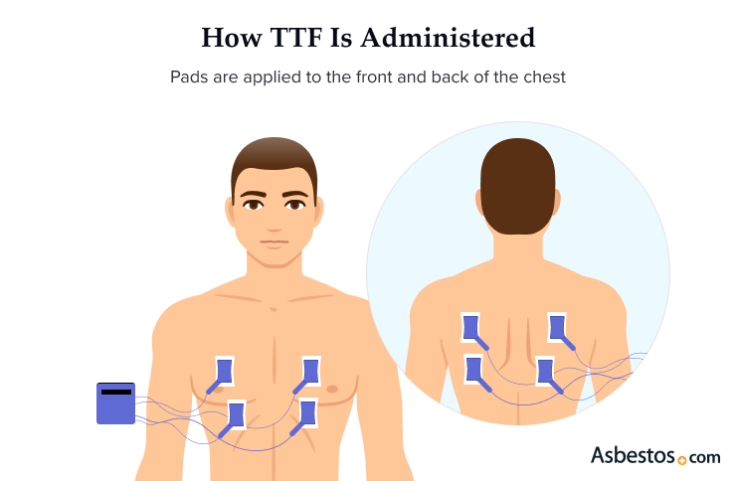
TTFields delivers electrical waves through insulated pads that stick to your skin. After your mesothelioma specialist prescribes TTFields therapy for you, a Novocure device support specialist will arrange for in-person training for you and your caregiver. Novocure’s TTFields delivery system is branded under Optune Lua and is a transportable device that can be used anywhere.
The system includes a TTFields-generating device, battery charger and rechargeable batteries, power supply, insulated pads, and a carrying bag. The electrical field’s frequency is 100 to 300 kilohertz, and the intensity is delivered at 1 to 3 volts per centimeter.
Your TTFields mesothelioma treatment is designed for continual use for 18 to 20 hours a day at home or on the go. You can run errands, take walks or do household chores while it’s administered. The carrying bag can be worn as a backpack or held in your hand. There are 4 pads attached to the front and back of your chest and back. The pads are changed every 2 to 3 days, and the area must be shaved. It involves no skin incisions or inserting any medical devices into the body.
Keeping the device charged can present some logistical challenges for active people. The delivery system is plugged into a power supply or uses a large rechargeable battery pack that must be carried to power the device. Every 3 weeks, people with mesothelioma will receive TTFields therapy with Alimta and cisplatin or carboplatin for 6 cycles. Research shows TTFields doesn’t increase the toxicity of chemo.
Skin redness and irritation are the most common side effects of TTFields therapy for mesothelioma. People may also experience side effects related to the chemotherapy they receive while prescribed TTFields.
Possible Side Effects of TTFields
Nearly 50% of patients who receive the therapy report skin irritation. Only 4% report a severe level of irritation. Discuss any side effects with your health care team if you notice new or worsening issues.
If you experience side effects from your mesothelioma treatment with TTFields, ask your doctor to recommend ways to manage them. Approaches to managing these effects are usually topical.
Avoiding and Managing TTFields Side Effects
It’s important to monitor your skin for signs of infection. These signs can include warmth, swelling or pus. Seek medical attention if you experience these effects.

We’ll get you the best doctor for your diagnosis and schedule appointments with them quickly.
Find My DoctorTTFields therapy for pleural mesothelioma is best for people in good overall health who can wear the device for 18 to 20 hours per day. People free of skin conditions such as infections, severe dermatitis or chest burns are more likely to be eligible for TTFields therapy.
People with an active implanted medical device, such as a pacemaker, may not be eligible. TTFields can interfere with the function of these medical devices. Pregnant women or those trying to conceive shouldn’t use TTFields. Its effects on fetal development aren’t well known.
For safety, only a mesothelioma doctor who has completed the required certification training can prescribe TTFields. Novocure’s training covers device operation, patient selection, potential side effects and troubleshooting.
TTFields therapy has shown promising results for people diagnosed with mesothelioma. The STELLAR trial, a pivotal clinical study, provided important data supporting its use alongside chemotherapy.
STELLAR Trial Data
Overall, these findings suggest TTFields could be a valuable addition to current treatment approaches. Researchers are also studying whether combining TTFields with other therapies, like immunotherapy, can lead to even better results. Continued research may expand how this therapy is used to help more patients in the future.
As of August 2024, TTFields therapy is available at more than 500 select cancer treatment centers across the country. Many additional centers are working to gain approval to offer this treatment. If you’re interested in TTFields, talk to your doctor to see if they can prescribe it and if you qualify for the therapy.
“We are always looking for treatments to improve survival, quality of life, and options for patients,” said Dr. Taylor Ripley, a thoracic surgeon and director of the Mesothelioma Treatment Center at Baylor College of Medicine. “This provides another therapeutic option that may be valuable.”
The cost of TTFields therapy can be significant, often ranging from $10,000 to $20,000 per month, depending on the region and health care system. The transportable delivery system, which delivers TTFields for mesothelioma patients, is a specialized medical device and its cost includes the device, contact pads and ongoing support.
Coverage may vary depending on the insurance provider and the patient’s specific plan. Prior authorization is required. Patient Advocates, your health care team and patient support from the manufacturer, Novocure, can help you navigate insurance coverage and financial assistance programs.
People with mesothelioma usually use TTFields continuously for 18 to 20 hours per day throughout their treatment course. The duration varies depending on your response to treatment and overall treatment plan.
In clinical studies, patients often used TTFields for 12 months or longer. Treatment continues as long as it’s tolerated and you benefit from it.
TTFields are most effective for treating pleural mesothelioma when used in combination with chemo, particularly Alimta (pemetrexed) and cisplatin. A mesothelioma specialist’s decision to use TTFields without chemo would depend on your specific case and treatment goals.
Stay up-to-date on treatment, research, clinical trials, doctors and survivors
The information on this website is proprietary and protected. It is not a substitute for professional medical advice, diagnosis or treatment. Any unauthorized or illegal use, copying or dissemination will be prosecuted. Please read our privacy policy and terms of service for more information about our website.
This website and its content may be deemed attorney advertising. Prior results do not predict a similar outcome.
The Mesothelioma Center’s claim as the most trusted resource is based on our more than 150 5-star Google and BBB reviews. Our organization also helps more than half of all mesothelioma patients annually diagnosed.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Asbestos.com. (2025, September 3). Tumor Treating Fields (TTFields) and Mesothelioma. Retrieved February 6, 2026, from https://www.asbestos.com/treatment/ttfields/
"Tumor Treating Fields (TTFields) and Mesothelioma." Asbestos.com, 3 Sep 2025, https://www.asbestos.com/treatment/ttfields/.
Asbestos.com. "Tumor Treating Fields (TTFields) and Mesothelioma." Last modified September 3, 2025. https://www.asbestos.com/treatment/ttfields/.

Dr. Andrea Wolf is the Director of the New York Mesothelioma Program at Mount Sinai in New York City. She focuses on multidisciplinary treatment, clinical research, community outreach and education.
Our fact-checking process begins with a thorough review of all sources to ensure they are high quality. Then we cross-check the facts with original medical or scientific reports published by those sources, or we validate the facts with reputable news organizations, medical and scientific experts and other health experts. Each page includes all sources for full transparency.
Please read our editorial guidelines to learn more about our content creation and review process.
