
Dr. William Breen is an innovative radiation oncologist in Rochester, Minnesota, at the much-acclaimed Mayo Clinic, a National Cancer Institute-designated Comprehensive Cancer Center for more than 40 years.
Brachytherapy is a type of radiation therapy that minimizes the damage done to healthy tissue, administering radiation via a small object placed inside the tumor. This allows the therapy to maintain a narrower focus on tumor growth.
Unlike traditional radiation therapy, brachytherapy uses no external radiation. Because of this defining property, it may also be referred to as internal radiation therapy, whereas the conventional type may be called external beam radiation therapy.
Internal radiation isn’t standard for lung cancer or mesothelioma, but researchers continue to test the treatment in clinical trials. Results from early studies show the potential for brachytherapy to extend life span and reduce the severity of symptoms. A March 2021 study reported thoracic cancer patients who receive brachytherapy benefit from fewer and less severe side effects after pulmonary surgery.
So far, it has been more effective in treating lung cancer. Researchers saw the best results for both types of cancer when they implemented a treatment called permanent brachytherapy. With this therapy, radioactive material is placed permanently into the tumor site and this material slowly loses its radioactivity over a few months.
Brachytherapy uses a radioactive material called an implant. The material may come in the form of a wire or a “seed” about the size of a grain of rice.
It may be placed during surgery with an intraoperative radiation therapy technique or it may be inserted into cancer tissue with a hollow tube. With IORT, the patient receives local or general anesthesia and the doctor typically uses an imaging scan such as a CT or ultrasound.
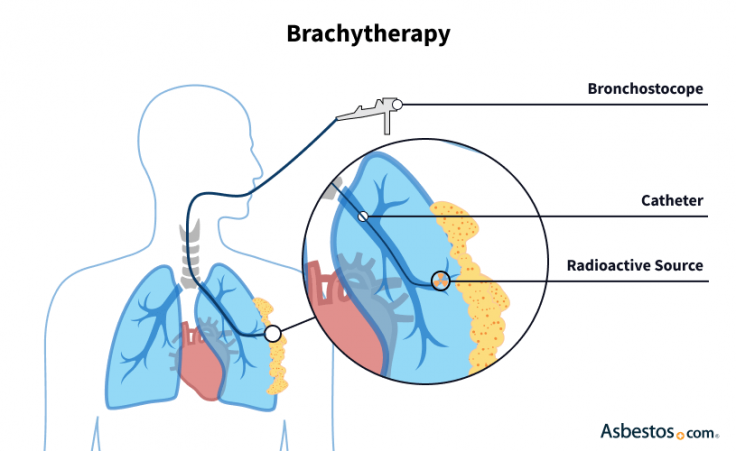
Once the implant is in place, it commonly only attacks tissue within a radius of 1cm. This can be more effective but requires precision.
IORT brachytherapy is always administered in conjunction with surgery, and some lung cancer patients receive standard brachytherapy after surgery to kill any remaining cancer cells. It may also be used alongside conventional radiation therapy to target tumor growth in multiple ways. It may be used as a potentially curative treatment to eradicate cancer or as a palliative treatment to reduce symptoms such as coughing and difficulty breathing, depending on the individual patient’s situation.

We’ll get you the best specialist for your diagnosis and schedule appointments with them quickly.
Find My DoctorThere are 2 primary types of brachytherapy: Low-dose rate and high-dose rate. People with lung cancer or mesothelioma usually receive a form of low-dose-rate treatment called permanent brachytherapy. This type continuously attacks the tumor with radiation for several months.
Depending on the type of therapy patients receive, they may undergo treatment for up to several weeks. This is shorter than the time needed to complete an external beam radiation therapy regimen, which can take up to 10 weeks.
Low-dose rate brachytherapy involves low doses of radiation for long periods. Radioactive material may be left in place for up to a week for this treatment.
The radiation can harm people nearby, so patients remain in the hospital while receiving LDR brachytherapy. Patients typically stay in private hospital rooms and can’t have guests for extended periods. LDR brachytherapy shouldn’t be painful or uncomfortable.
This type of LDR involves permanently leaving seed implants in the body. This is the most common type used to treat mesothelioma and lung cancer because it has the most improved patient survival rates in clinical trials.
Typically, the implant is inserted during surgery as a form of IORT. Radioactive seeds are woven into a flexible mesh and stitched into place during a surgical procedure such as pneumonectomy or pleurectomy/decortication. This type of brachytherapy, designed to kill cancer cells that can’t be removed with surgery alone, actively emits radiation for about 3 months. Seeds may be mildly radioactive for another year. The inactive seeds then remain in the body permanently.
High-dose-rate brachytherapy involves high doses of radiation for a maximum of 20 minutes. The patient receives HDR treatment once or twice daily for several days or weeks. After the physician inserts the seed via a tube, they typically leave the room while the radioactive material is in place. It doesn’t require a hospital stay and, like LDR brachytherapy, isn’t painful or uncomfortable.

Try our new clinical trials search tool to find active trials near you. Get help enrolling today.
Find a Clinical TrialThe most common side effect is tenderness at the site of insertion, which subsides after a few months. Patients may also experience temporary swelling at the treatment site. Due to the sharp radiation dose fall-off with brachytherapy, it may be associated with fewer side effects than external beam radiation therapy in some cases.
Regardless of cancer type, brachytherapy can also cause skin irritation, such as redness, dryness, sensitivity or darkening under the breast or arm. In more severe cases, the skin may peel or develop moist ulcers. Doctors can prescribe prescription ointments that help relieve many of these side effects.
Most side effects usually go away after treatment ends, but you may feel very tired for 4 to 6 weeks after your last treatment. If symptoms worsen or don’t resolve with medications, your oncologist may refer you to a specialist to diagnose or further treat the problem.
Stay up-to-date on treatment, research, clinical trials, doctors and survivors
The information on this website is proprietary and protected. It is not a substitute for professional medical advice, diagnosis or treatment. Any unauthorized or illegal use, copying or dissemination will be prosecuted. Please read our privacy policy and terms of service for more information about our website.
This website and its content may be deemed attorney advertising. Prior results do not predict a similar outcome.
The Mesothelioma Center’s claim as the most trusted resource is based on our more than 150 5-star Google and BBB reviews. Our organization also helps more than half of all mesothelioma patients annually diagnosed.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2025, June 23). Brachytherapy for Mesothelioma. Asbestos.com. Retrieved December 18, 2025, from https://www.asbestos.com/treatment/radiation/brachytherapy/
Selby, Karen. "Brachytherapy for Mesothelioma." Asbestos.com, 23 Jun 2025, https://www.asbestos.com/treatment/radiation/brachytherapy/.
Selby, Karen. "Brachytherapy for Mesothelioma." Asbestos.com. Last modified June 23, 2025. https://www.asbestos.com/treatment/radiation/brachytherapy/.

Dr. William Breen is an innovative radiation oncologist in Rochester, Minnesota, at the much-acclaimed Mayo Clinic, a National Cancer Institute-designated Comprehensive Cancer Center for more than 40 years.
Our fact-checking process begins with a thorough review of all sources to ensure they are high quality. Then we cross-check the facts with original medical or scientific reports published by those sources, or we validate the facts with reputable news organizations, medical and scientific experts and other health experts. Each page includes all sources for full transparency.
Please read our editorial guidelines to learn more about our content creation and review process.
