Photodynamic Therapy for Mesothelioma
Photodynamic therapy is a newer mesothelioma treatment that uses light energy to kill cancer cells. Treatment involves injecting a photosensitizer drug into the patient before surgery. When the cells are exposed to light, it activates the drug to kill the cancer cells.
What Is Photodynamic Therapy?
Photodynamic therapy is a cancer treatment that uses a special drug and light to kill mesothelioma cells. The drug is light sensitive. It absorbs light and causes a reaction. PDT targets tumors without hurting surrounding tissue, which can help reduce the side effects you may experience.
One of the most commonly used photosensitive drugs is Photofrin, or porfimer sodium. The U.S. Food and Drug Administration hasn’t yet approved it for treating mesothelioma, but it’s approved for other cancers. Photofrin builds up in cancer cells, making them easier to destroy when exposed to light.
This therapy is most often used as part of a combination of treatments for pleural mesothelioma. Researchers continue to explore how PDT can improve outcomes for people with mesothelioma.
How Does Photodynamic Therapy Work for Mesothelioma?
Photodynamic therapy uses a photosensitizing drug that builds up in mesothelioma cells, making them sensitive to light. A surgeon then activates the drug during surgery with a special laser. This process triggers a reaction that destroys the cancer cells while preserving healthy tissue. Doctors may combine PDT with other mesothelioma treatment options.
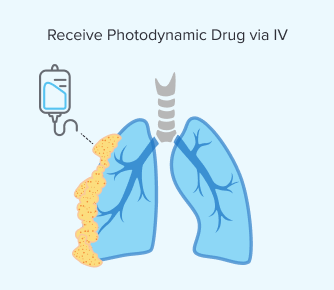
Step 1: Receiving the Photodynamic Drug
You would receive a light-sensitive drug, such as Photofrin, via an IV. This allows it to circulate throughout your body. Sometimes these drugs can be given through the skin but not for mesothelioma tumors, which are located internally.
Similar to chemotherapy, both healthy and cancerous cells can absorb this drug. However, the photodynamic medicine stays longer in mesothelioma cells.
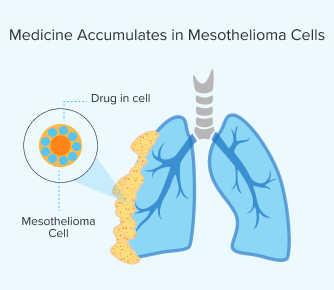
Step 2: Medicine Accumulates in Mesothelioma Cells
After the drug enters your bloodstream, the medicine builds up in the mesothelioma cells in your body. The time this takes varies per person. It usually takes between a few hours and days. Your metabolism and the type of drug you’re given can affect how long it will take.
You can go home after treatment, but you’ll have to stick to strict light exposure guidelines such as avoiding direct sunlight and bright indoor lighting to prevent skin sensitivity. Once enough of the drug has built up in mesothelioma cells, light is applied during surgery.
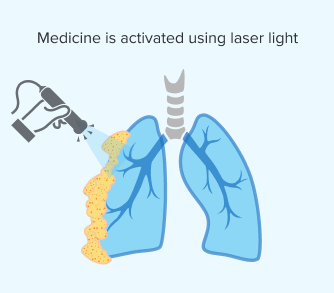
Step 3: Applying Light
Once the medicine has sufficiently accumulated, your doctor will activate it using a specific wavelength of laser light. This makes sure the light reaches the targeted tumors directly during surgery. Applying the light is critical because it triggers a chemical reaction that destroys cancer cells.
The effectiveness of PDT depends on light reaching your mesothelioma tumors. In pleural mesothelioma, doctors can directly target tumors on the lung’s lining. But with peritoneal mesothelioma, it’s harder to apply light in the abdominal cavity. Researchers are working on new ways to deliver light for different mesothelioma types.
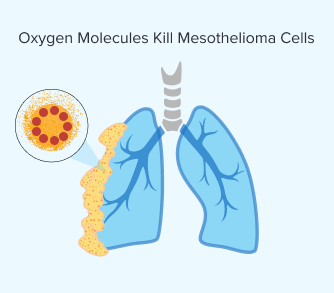
Step 4: Oxygen Molecules Kill Mesothelioma Cells
Once the light is applied and the photosensitizing drug is activated, the chemical reaction produces a highly reactive form of oxygen. The volatile oxygen that results from this process destroys mesothelioma cells.
This reaction can also damage blood vessels that supply the tumor. This further hinders the ability of the mesothelioma tumor to grow and spread. Over time, your body clears dead cancer cells and begins healing.
Benefits of Photodynamic Therapy for Mesothelioma
Photodynamic therapy is promising for mesothelioma, targeting cancer cells while minimizing damage to healthy tissue. This precision reduces the side effects you might experience compared to more aggressive treatments like chemo or radiation.
PDT is most effective when used during surgery, when doctors can directly expose tumors to light. Some patients report mild side effects. Overall, PDT offers a targeted approach with fewer complications.
How Photodynamic Therapy Helps Mesothelioma Patients
- Enhances survival rates: Studies indicate combining PDT with surgery may improve mesothelioma survival rates.
- Minimally invasive: PDT doesn’t require additional incisions or major procedures beyond light application during surgery.
- Preserves healthy tissue: Unlike chemo, PDT targets only the cancer cells, reducing harm to surrounding tissues.
- Reduces side effects: People typically experience fewer whole-body or systemic side effects compared to traditional cancer treatments.
A University of Pennsylvania study shows PDT boosts mesothelioma survival. People who underwent lung-sparing surgery and PDT had a median survival of 31.7 months. People who specifically have the epithelial subtype of mesothelioma reached 41.2 months.
Another study in the Annals of Thoracic Surgery found patients receiving surgery and PDT had a median survival of 13 to 14 months versus 10 months for surgery alone. These results highlight PDT’s role in improving mesothelioma survival rates.

You shouldn’t have to choose between getting care and paying for it. Get the financial support you deserve.
See My OptionsSide Effects and Limitations of Photodynamic Therapy
While PDT offers a targeted approach to treating mesothelioma, it has some side effects. These include temporary skin and eye sensitivity to light, localized swelling and discomfort. Fatigue is also common as the body recovers from the procedure, and the immune system processes the destroyed cancer cells.
Possible PDT Side Effects
- Difficulty swallowing or breathing: Mild irritation may occur when PDT is used near the throat or lungs.
- Fatigue: Some patients experience tiredness following the procedure.
- Inflammation and redness: Treated areas may become temporarily inflamed.
- Localized swelling and pain: Temporary discomfort may occur at the treatment site.
- Skin and eye sensitivity to light: Patients must avoid bright lights and direct sunlight for several weeks after treatment.
PDT is only practical for surface tumors or those exposed during surgery. It’s usually used along with other therapies. It’s not very effective when mesothelioma has spread extensively. Despite its limitations, PDT is a promising treatment for mesothelioma.
Who Is Eligible for Mesothelioma Photodynamic Therapy?
Your disease spread must be limited to be considered a good candidate for photodynamic therapy for mesothelioma. People in stages 1-3 are generally more eligible. PDT isn’t practical for mesothelioma tumors deeply embedded in tissue or those that have spread far throughout the body.
It’s important to also be a good candidate for surgery since light is applied directly to tumors. Factors that affect your eligibility for PDT and surgery include your overall health, lung function and what treatments you may have already had.
PDT is most beneficial for people with pleural mesothelioma and select cases of peritoneal mesothelioma, where doctors can access the cancerous tissue during surgery. Most candidates for PDT undergo lung-sparing surgery or cytoreductive surgery. In these cases, doctors remove visible tumors before applying light treatment.
What to Expect During Photodynamic Therapy Recovery
Your recovery time from photodynamic therapy for mesothelioma varies depending on your overall health and the extent of your surgery. Most people stay in the hospital for a few days to a week, depending on their procedure and response to treatment.
PDT itself doesn’t cause significant whole-body side effects. However, people often experience fatigue, mild discomfort and skin sensitivity from the photosensitizing drug.
Tips for Managing Effects of PDT Treatment
- Manage pain and discomfort: Over-the-counter pain relievers like acetaminophen or ibuprofen can help with mild discomfort. If the burning or itching is severe, your doctor may prescribe stronger medicine.
- Protect your skin from light sensitivity: Avoid direct sunlight and bright indoor lights for at least 4 to 6 weeks. Sunscreens generally aren’t advised, as many contain chemicals that can irritate sensitive skin. Wear protective clothing, sunglasses and wide-brimmed hats outside.
- Be cautious with skin remedies: While natural skin soothers like aloe vera and vitamin E may seem helpful, check with your doctor first. Lidocaine-based burn gels may provide relief, but some people experience irritation.
- Stay hydrated and support healing: Drinking plenty of water can help your body clear the photosensitizing drug. Avoid alcohol and smoking, which can slow recovery and increase inflammation.
- Know when to resume normal activities: Regaining energy levels may take several weeks. You should ease back into daily activities and wait until your doctor clears you for strenuous exercise.
Most side effects of PDT fade within a few weeks, but some patients’ sensitivity to light can last up to 3 months. Following recovery guidelines and staying in touch with a mesothelioma specialist can help you safely navigate your healing process and maximize the benefits of PDT.
Clinical Trials for Mesothelioma Photodynamic Therapy
Photodynamic therapy is a newer treatment for mesothelioma. Emerging research has shown promising results, particularly when PDT is part of combination or multimodal treatment plans.
Current Trials Studying PDT and Mesothelioma
- Combination of photodynamic therapy and surgery: This trial explores the use of PDT with radical pleurectomy surgery to treat pleural mesothelioma. Patients receive a light sensitive drug before surgery and then light treatment during surgery.
- Photodynamic therapy for peritoneal mesothelioma: A clinical trial is assessing how well PDT works for people with peritoneal mesothelioma and how safe it is for them. The study involves giving a photosensitizing drug and then light exposure during surgery to target abdominal tumors.
- Photodynamic therapy with immunotherapy: Researchers are studying combining PDT with immunotherapy. This approach aims to integrate the 2 advanced therapies to improve patient outcomes.
In 2024, researchers developed a new system to measure light and drug levels during PDT, helping doctors improve treatment accuracy. Studies also show that low-dose PDT may help the immune system fight mesothelioma, making tumors easier to target.
If you’re interested in participating in a PDT clinical trial for mesothelioma, consult a Patient Advocate or oncologist to determine your eligibility. You can also research active trials on resources like the National Library of Medicine and reach out to trial coordinators for enrollment details.
Common Questions About Mesothelioma Photodynamic Therapy
- How does photodynamic therapy compare to radiation therapy for mesothelioma?
-
Photodynamic therapy uses a drug and light to directly destroy mesothelioma cells. This targets them precisely while reducing damage to healthy tissue. In contrast, radiation therapy delivers high-energy beams that can harm both cancerous and normal cells, typically used after surgery or alongside chemotherapy.
- Can photodynamic therapy be repeated if mesothelioma returns?
-
Doctors may repeat PDT if mesothelioma returns, but it depends on your health and how well your cancer responds. Some people may need different treatments, like surgery or chemo. A mesothelioma specialist can help decide if another round of PDT is a good option.
- Where is photodynamic therapy for mesothelioma available?
-
Leading cancer centers like Abramson, Stanford and Roswell Park offer PDT for mesothelioma. These facilities incorporate PDT into broader treatment plans to enhance survival. You should consult your doctor about finding PDT and qualifying for treatment.