Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

CTLA-4 is a protein that plays an important role in the immune system. It helps keep the immune system in check by regulating T cells. Ipilimumab (Yervoy) and tremelimumab are two immune checkpoint inhibitor drugs that block CTLA-4 to allow T cells to find and fight cancers such as mesothelioma.
Cytotoxic T-lymphocyte-associated protein 4, or CTLA-4, is a protein on the surface of T cells that functions as an immune checkpoint. It affects how T cells recognize cancer cells.
T cells attack foreign invaders, such as viruses and cancer cells.
CTLA-4 prevents T cells from attacking healthy parts of the body, but it can also prevent T cells from identifying and killing cancer cells.
Drugs that block CTLA-4 are called immune checkpoint inhibitors. These drugs have the potential to help people live longer with mesothelioma cancer.
Targeting CTLA-4 with immune checkpoint inhibitors is a form of immunotherapy. New immunotherapies are among the most promising mesothelioma treatments in development right now.
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

French researchers discovered CTLA-4 in 1987. Nearly a decade later, scientists at the University of California, Berkley, realized the role of CTLA-4.
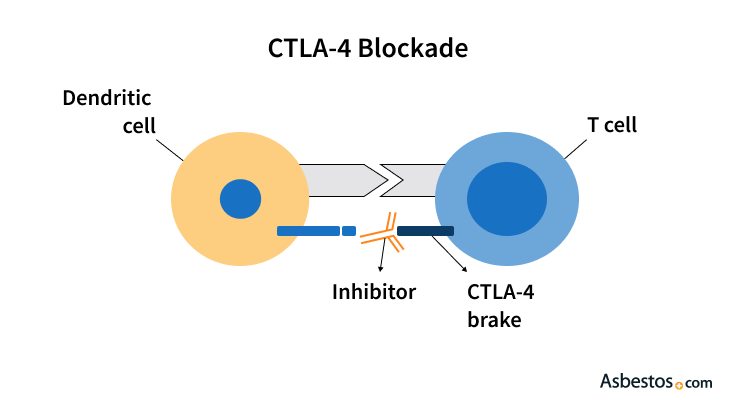
The protein acts as a break, stopping T cells from attacking cancer cells. Researchers call this breaking mechanism an immune checkpoint.
Immune checkpoints work like on and off switches, activating or deactivating the parts of the immune system.
When activated, CTLA-4 downregulates the immune system by stopping T cells from finding cancer cells. In 2021, a clinical research study concluded that patients who express higher levels of CTLA-4 may have decreased mesothelioma survival times.
Scientists have developed drugs to block and deactivate CTLA-4 — a process commonly called a CTLA-4 blockade — which allows T cells to find and attack cancer cells. These drugs are known as immune checkpoint inhibitors.
Other immune checkpoint inhibitors targeted in mesothelioma treatment include PD-1 and PD-L1. Drugs that target these checkpoints include Keytruda (Pembrolizumab), Opdivo (Nivolumab) and Imfinzi (Durvalumab).
Two checkpoint inhibitor drugs work against CTLA-4: Yervoy and tremelimumab.
Yervoy is FDA-approved to treat late-stage melanoma. Tremelimumab is not FDA-approved, but in 2015 it received orphan drug designation to encourage research into it.
Most of the mesothelioma clinical trials involving Yervoy and tremelimumab combine them with immune checkpoint inhibitors that target other proteins. Studies show that targeting PD-1 and CTLA-4 together increases effectiveness.
Mesothelioma researchers use the combination approach because it has proven more effective in melanoma treatment. For example, when used alone, about 11 percent of melanoma patients respond to Yervoy.
When the anti-PD-1 drug Opdivo is added to Yervoy, about 61% of melanoma patients respond.
Researchers found that adding Opdivo to Yervoy increases the response rate in mesothelioma patients. 2017 French researchers announced a significant improvement in response rates at the 2017 ASCO Annual Meeting.
Approximately 44% of mesothelioma patients had control of their cancer with Opdivo alone, while 50% had control of their cancer using Yervoy combined with Opdivo.
These results justified a phase III trial of Yervoy and Opdivo in mesothelioma patients, which began in 2017.
Less research is available on tremelimumab than Yervoy, but preliminary results show further research is warranted.
Clinical trials suggest that Yervoy and tremelimumab are effective for some people with mesothelioma. Further research is necessary to determine which mesothelioma patients may benefit the most from immune checkpoint inhibitors that target CTLA-4.
Additional research is also required to see which drug combinations work best among people with mesothelioma.
Recommended ReadingYour web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2025, April 8). CTLA-4 and Mesothelioma. Asbestos.com. Retrieved April 14, 2025, from https://www.asbestos.com/treatment/immunotherapy/ctla4/
Selby, Karen. "CTLA-4 and Mesothelioma." Asbestos.com, 8 Apr 2025, https://www.asbestos.com/treatment/immunotherapy/ctla4/.
Selby, Karen. "CTLA-4 and Mesothelioma." Asbestos.com. Last modified April 8, 2025. https://www.asbestos.com/treatment/immunotherapy/ctla4/.
Dr. Andrea Wolf is the Director of the New York Mesothelioma Program at Mount Sinai in New York City. She focuses on multidisciplinary treatment, clinical research, community outreach and education.
Our fact-checking process begins with a thorough review of all sources to ensure they are high quality. Then we cross-check the facts with original medical or scientific reports published by those sources, or we validate the facts with reputable news organizations, medical and scientific experts and other health experts. Each page includes all sources for full transparency.
Please read our editorial guidelines to learn more about our content creation and review process.
