
Connect with top-rated doctors specializing in mesothelioma treatment, who will personalize treatment options based on your diagnosis.
Find Your DoctorChemotherapy and immunotherapy are both systemic treatments for cancer that work by distributing certain drugs throughout the body. When recommending immunotherapy vs. chemotherapy to treat mesothelioma, specialists consider the individual patient as well as the type and stage of the disease.

Immunotherapy is a type of cancer treatment that helps the body’s immune system recognize and kill mesothelioma cells. Normally, the immune system defends the body against harmful invaders like bacteria and viruses. But cancer cells can hide or disguise themselves to avoid detection. Immunotherapy makes it easier for the immune system to identify and target cancer cells.
Immunotherapy can be used alone or with other treatments to improve outcomes. This targeted approach can lead to longer-lasting results for some people with mesothelioma, potentially controlling cancer longer than other therapies.
Chemotherapy for mesothelioma uses strong drugs to fight cancer cells in the body. Cancer cells grow and divide much faster than normal cells.
Chemotherapy doesn’t only affect cancer cells. It also impacts other fast-growing cells. Hair, skin and the lining of the stomach are affected. That’s why people on chemo might experience side effects like hair loss, nausea and fatigue. The drugs are often given in cycles, with breaks in between to help healthy cells recover.
Chemo blocks a mesothelioma tumor’s ability to grow and spread. Immunotherapy boosts your body’s ability to kill cancer cells. Both therapies can affect the entire body (systemic therapy). Each treatment has a different way of attacking cancer cells.
Peritoneal mesothelioma survivor Tami Pream has been undergoing heated chemo treatments, or HIPEC, combined with an immunotherapy regimen. She tells us her treatment combinations have been successful so far. “It seems to be making somewhat of a difference in how I’m feeling,” Tami says.
Most chemo drugs damage cancer cells as they divide and multiply. They prevent the synthesis of DNA and other molecules. These are vital for cell division and survival. Cancer cells grow and divide faster than most healthy cells. So they’re an ideal target for chemo drugs.
Immunotherapy drugs can help your immune system spot cancer cells. Cancer cells come from your own cells. So your immune system doesn’t always recognize them as a threat. Boosting the immune system helps the body kill cancer cells.
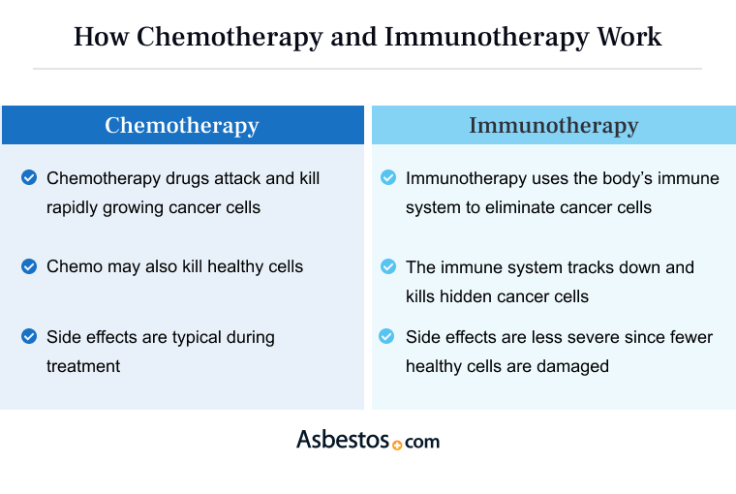
Immunotherapy is more targeted than chemotherapy. Chemotherapy targets all rapidly dividing cells, impacting both cancerous and healthy cells. Immunotherapy targets only cancer cells, enhancing the immune system’s ability to recognize and attack them. Immunotherapy trains immune cells to identify cancer as a threat, leaving healthy cells largely unaffected.
Chemo and immunotherapy have shared and unique side effects. Chemo’s targeting of rapidly dividing cells affects healthy cells in areas like the digestive tract and hair follicles. Symptoms like nausea, vomiting and hair loss may occur.
Immunotherapy’s impact on the immune system causes immune-related side effects. Inflammation can develop in the lungs, skin, liver or intestines. Immune side effects occur less frequently than chemo side effects, but they may require immunosuppressive treatment when severe.

Connect with top-rated doctors specializing in mesothelioma treatment, who will personalize treatment options based on your diagnosis.
Find Your DoctorImmunotherapy and chemo offer improved survival and quality of life benefits for people with mesothelioma. Chemo helps people who need immediate symptom relief. Immunotherapy potentially offers longer-lasting effects.
Chemo is widely accessible, making it a common first-line treatment. Many forms of immunotherapy have also become widely accessible. These therapies may be used interchangeably as both first- and second-line treatments.
Immunotherapy for mesothelioma harnesses the body’s immune system to fight cancer more effectively. It helps the immune system recognize and target mesothelioma cells. Certain types of immunotherapies may train the body to recognize cancer cells for a long time.
For some people, immunotherapy results in long-lasting responses. The effect can last months or even years after treatment. Mesothelioma survivor Andy Ashcraft lived for several years with stage 4 mesothelioma thanks to immunotherapy. The drug amatuximab, which he received through a clinical trial, was effective for 3 years.
Immunotherapy is beneficial for patients with advanced or inoperable mesothelioma when conventional treatments may be less effective. It can boost survival and quality of life, improving symptoms.
Chemotherapy can shrink mesothelioma tumors and control their spreading. Chemo drugs target and kill cancer cells, reducing tumor size. Shrinking tumors alleviates symptoms like pain and shortness of breath. Its ability to limit cancer spread helps slow disease progression. These factors make it an effective first-line treatment for patients needing quick relief or when surgery isn’t feasible.
Chemo’s tumor-shrinking effect can enhance the effectiveness of other treatments. For example, it can reduce tumor size before surgery. This has the potential to make surgical tumor removal easier. Chemo can also be combined with immunotherapy, targeted therapy and Tumor Treating Fields. Combining chemo with these therapies enhances effectiveness.
Chemo and immunotherapy for mesothelioma patients have many of the same side effects, including fatigue and flu-like symptoms. Drug interactions can make it hard to treat side effects. Some medications can reduce how well chemo and immunotherapy work.
Both treatments for mesothelioma can have serious side effects. Chemotherapy can cause severe infections. It can cause long-term memory problems and “chemo brain” or “brain fog.” Immunotherapy can cause pneumonia, hepatitis, kidney issues and organ damage.
Immunotherapy drugs usually have milder side effects. Inflammation, for example, can cause side effects as part of the body’s immune response. This can lead to symptoms such as fever and fatigue. These side effects are usually reversible.
Chemo drugs target cells that divide rapidly. This includes cancer cells and some healthy cells, like hair cells, the lining of the gut and bone marrow. This is why some chemo drugs cause hair loss, mouth sores and nausea.
Your medical team can prescribe medication and therapies to help alleviate side effects. Talk to your doctor as soon as side effects develop. Prompt treatment can help keep side effects under control.
| Chemotherapy Side Effects | Immunotherapy Side Effects | Shared Side Effects |
|---|---|---|
| Cognitive problems | Abnormal blood pressure | Constipation, diarrhea, nausea & vomiting |
| Infections | Dizziness | Difficulty breathing |
| Hair loss | Injection site reaction | Infection |
| Low blood counts | Weakness | Mouth sores and skin irritation |
| Fatigue, fever and chills | Fatigue, fever and chills | Fatigue, fever and chills |
| Pain and headaches | Pain and headaches | Pain and headaches |
| Weight loss | Weight loss | Weight loss |
Side effects of both therapies usually begin soon after treatment starts. Sometimes, they can last for months or years after treatment. But they usually stop soon after treatment ends.
Chemo side effects vary with different drugs. Immunotherapy side effects are often less predictable than those of chemo. Cancer treatments affect everyone differently.
Trials have compared the effects of immunotherapy with chemotherapy for people with mesothelioma. In first-line treatment, immunotherapy shows a slight survival benefit over chemo. Immunotherapy didn’t show significant survival benefit in second-line treatment. Combining certain chemo and immunotherapy drugs can improve effectiveness as well.
Chemo and immunotherapy are first-line treatments for advanced pleural mesothelioma. This includes stage 2 or higher and inoperable stage 1. A combo of immunotherapy drugs Opdivo and Yervoy is often used. Keytruda may be used alone or in combination with chemotherapy.
When Are Immunotherapy and Chemo Most Effective?
Chemotherapy drugs are also combined. Alimta with either cisplatin or carboplatin is often given before surgery to remove pleural tumors. Dr. Jeffrey Velotta, a thoracic surgeon at Kaiser Permanente Oakland Medical Center, tells us pleural mesothelioma patients live the longest when they undergo surgery first, then chemo and then immunotherapy.
Doctors often treat peritoneal mesothelioma with surgery and heated chemo. This type of chemo is localized and performed during surgery. Immunotherapy has rarely been used in peritoneal cases, but research is ongoing.
Research published in the journal Cancers shows that for some cancers, a combo of immunotherapy and chemo is better than either alone. If cancer recurs after using only one therapy, doctors may then use a mix of the two.
The BEAT-meso and DREAM3R trials are testing new drug combinations for mesothelioma. These combinations use immunotherapy and chemotherapy. Ongoing clinical trials offer access to the latest in cancer treatment. Discuss open trials with your mesothelioma doctor.
The main factors for chemo or immunotherapy eligibility are health and cancer type. Some therapies may not be suitable for those with heart problems or serious health issues.
Some patients qualify for both therapies. Mesothelioma survivor Barbara Lapalla tells us she received immunotherapy after chemo stopped working. Lapalla says, “I tell people today, I’m a walking miracle. They don’t believe in miracles. Then they see me.”
Underlying conditions can limit therapeutic options. For example, people with severe heart issues may not be eligible for chemo. A person with an autoimmune disease may not qualify for immunotherapy.

Discover financial assistance programs, insurance guidance, and veteran benefits to help cover your mesothelioma treatment expenses.
Explore Your OptionsMesothelioma immunotherapy is generally much more expensive than chemo. Its history of use in cancer treatment often makes chemo more accessible. Immunotherapy’s high cost can limit accessibility, though some insurance plans do cover it for mesothelioma.
Health insurance, medical grants and other types of financial aid help patients afford treatment. Veterans may qualify for VA health care and disability compensation. Social Security Disability is available for anyone diagnosed with mesothelioma. Asbestos lawsuits and trust fund claims also help patients pay for medical care.
Immunotherapy for mesothelioma is generally quite expensive. The combination of Yervoy and Opdivo for mesothelioma can cost $256,000 per year. The specific price depends on factors like the type of immunotherapy drug, treatment frequency and health care facility charges.
Some therapies, like CAR T-cell therapy, can be even more costly. Some patients qualify to receive these drugs through clinical trials. Although many insurance plans offer some coverage, high out-of-pocket costs often remain. Financial aid options and pharmaceutical assistance programs can help patients afford immunotherapy.
The cost of chemo can vary a lot depending on which drugs are used and how long or often treatments last. A 2013 report put the price of first-line chemo for mesothelioma at $40,102 with Alimta and cisplatin. That was 12 years ago and the cost was already staggering.
Most insurance plans cover chemo, but the amount they cover can vary for each patient. Chemo is often easier to access than immunotherapy because it has been used for a much longer time.
Health insurance plays a key role in covering the high costs of cancer treatments like chemo and immunotherapy. Most insurance plans offer some coverage for these treatments, including private health insurance, Medicare and Medicaid.
However co-pays, deductibles and how much of the costs are covered can vary greatly depending on the plan. Even with coverage, these treatments can still be expensive for many patients.
Talking with Patient Advocates and financial counselors can help people find financial assistance. Programs like medical grants can reduce the financial burden. Veterans with mesothelioma can also file VA claims. Seeking financial help allows patients to concentrate on recovery without worrying about treatment costs.
The cost of immunotherapy vs. chemotherapy varies. It depends on the treatment length, cancer type and its stage. Chemo is usually less expensive than immunotherapy. A 2020 study on non-small cell lung cancer found chemotherapy cost $147,801 and immunotherapy $202,202 in 2016.
Insurance doesn’t always cover all cancer treatment costs. Patient Advocates at The Mesothelioma Center can help you find financial aid.
Chemotherapy and immunotherapy vary in how long they last. It depends on the cancer stage, type and side effects. Immunotherapy for advanced pleural mesothelioma usually lasts 2 years. Chemotherapy for pleural mesothelioma usually lasts for 5 months.
Doctors use chemo or immunotherapy for epithelioid pleural mesothelioma. Immunotherapy is recommended for biphasic or sarcomatoid pleural mesothelioma. Chemo is approved for peritoneal mesothelioma. Immunotherapy isn’t approved yet for this type.
Yes, mesothelioma treatment depends on the stage. People with early-stage disease may be eligible for surgery with chemo and/or radiation. However, those with advanced disease often don’t qualify for surgery. The first-line treatment is systemic therapy.
Stay up-to-date on treatment, research, clinical trials, doctors and survivors
The information on this website is proprietary and protected. It is not a substitute for professional medical advice, diagnosis or treatment. Any unauthorized or illegal use, copying or dissemination will be prosecuted. Please read our privacy policy and terms of service for more information about our website.
This website and its content may be deemed attorney advertising. Prior results do not predict a similar outcome.
The Mesothelioma Center’s claim as the most trusted resource is based on our more than 150 5-star Google and BBB reviews. Our organization also helps more than half of all mesothelioma patients annually diagnosed.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Asbestos.com. (2026, February 9). Immunotherapy vs. Chemotherapy. Retrieved March 5, 2026, from https://www.asbestos.com/treatment/immunotherapy-vs-chemotherapy/
"Immunotherapy vs. Chemotherapy." Asbestos.com, 9 Feb 2026, https://www.asbestos.com/treatment/immunotherapy-vs-chemotherapy/.
Asbestos.com. "Immunotherapy vs. Chemotherapy." Last modified February 9, 2026. https://www.asbestos.com/treatment/immunotherapy-vs-chemotherapy/.

Dr. Landau is the Medical Director of Virtual Hematology at the Medical University of South Carolina, where he leads programs that expand access to cancer care through telehealth. With more than 18 years of experience in oncology and hematology, he specializes in hematologic and genitourinary cancers, including bladder, prostate and kidney cancers. He has held multiple leadership roles in cancer program development and previously served as section chief of hematology and oncology at Orlando Health UF Health Cancer Center, where he founded its telehealth program.
Our fact-checking process begins with a thorough review of all sources to ensure they are high quality. Then we cross-check the facts with original medical or scientific reports published by those sources, or we validate the facts with reputable news organizations, medical and scientific experts and other health experts. Each page includes all sources for full transparency.
Please read our editorial guidelines to learn more about our content creation and review process.
