New Mesothelioma Treatments
New mesothelioma treatments include emerging and enhanced standard therapies. Some vaccine and gene therapies, for example, aim to boost immunotherapy, a first-line treatment for pleural mesothelioma. New combinations of chemotherapy and immunotherapy also show promise in extending survival.
What Are the New Treatments for Mesothelioma?
Experimental new treatment options for mesothelioma include advances such as cancer vaccines, enzyme therapy, gene therapy and virotherapy. These promising treatments may provide hope for patients who don’t respond to standard therapies.
Traditional treatments help people with mesothelioma cancer live longer with a better quality of life. However, there is still much room for improvement. Recent research has also led to several emerging treatments for mesothelioma.
We don’t yet have a cure for mesothelioma, but doctors have developed new treatments in recent years that have improved survival. Emerging mesothelioma treatments have identified new targets against mesothelioma cancer.
Many of these new treatments work best when paired with traditional options. For example, cancer vaccines can improve the treatment response rate of other immunotherapies.
- Enzyme therapy has been hailed as the most significant breakthrough in decades. Compared to standard chemotherapy, ADI-PEG20 or pegargiminase, for example, quadrupled survival at 36 months.
- In one study, adding the UV1 mesothelioma vaccine nearly doubled the response rate to Opdivo and Yervoy immunotherapy.
- The GPS (galinpepimut-S) vaccine led to stable disease in a third of patients, with a tumor decrease of 17% during clinical trials.
- Gene therapy that replaces a defective BAP1 gene leads to tumor suppression, and transferring “suicide genes” into cancer cells can improve some chemotherapies.
Anti-Angiogenic Drugs
A type of medication known as anti-angiogenic drugs target the growth of blood vessels in tumors to treat mesothelioma. Tumors need constant oxygen and nutrients to grow and spread. New blood vessels help them achieve that.
Anti-angiogenic drugs stop the growth of these blood vessels, which can prevent tumor spread. One example is bevacizumab (Avastin), but it can cause serious side effects such as blood clots, hemorrhaging and low white blood cell counts.
Cryotherapy
Cryotherapy is a type of cancer treatment that uses freezing temperatures to kill cancer cells. This treatment can help reduce the size of tumors and improve symptoms of mesothelioma.
As a targeted therapy, cryotherapy only targets the localized areas of the body affected with cancer. This means it’s less invasive and has fewer side effects than other treatments. A recent study showed cryotherapy helped relieve mesothelioma patients’ chest pain for about 5 weeks.
Epigenetic Therapy
Epigenetic therapy is a way to treat mesothelioma that focuses on how our DNA responds to factors such as age, environment and lifestyle. Targeting specific parts of our DNA, including UHRF1 and inflammation biomarkers, may help reverse the damage asbestos causes and promote healthy cell growth.
A recent study showed these therapies can improve survival rates for patients with pleural mesothelioma. Researchers are currently testing a new drug called tazemetostat (Tazverik) in clinical trials to see if it can help regulate epigenetic changes and improve outcomes for mesothelioma patients.

Some of the most exciting technologies for mesothelioma cancer care involve DNA, gene manipulation or gene therapy. Genomic sequencing is going to be the future of personalized medicine.
Gene Therapy
The goal of gene therapy is to fix or replace the faulty genes that lead to cancer. All cells in the body have DNA. Sometimes changes in this DNA cause normal cells to turn into cancerous tumors. Gene therapies work in different ways to address this.
Gene transfer involves adding healthy genes to cancer cells. For example, replacing a gene called BAP1 can help stop the tumors from growing. Another approach is to add “suicide genes” to the cancer cells. These genes make the cells more sensitive to chemotherapy drugs.
Photodynamic Therapy
Photodynamic therapy is a cancer treatment that involves injecting drugs that react to a special kind of light. Shining this light on cancer cells during surgery activates the drug, killing the cells to stop the cancer from spreading.
This treatment is especially promising because it has few side effects, which means patients can have a better quality of life while being treated. Researchers have studied low-dose photodynamic therapy (L-PDT) combined with immunotherapy in mice. The results showed complete mesothelioma regression in 37.5% of animal test subjects.
Vaccine Therapy
New therapies for mesothelioma include vaccines that teach the body’s immune system how to fight cancer cells. These treatments work like the flu vaccine that helps your body fight the flu virus.
The mesothelioma vaccine is still in clinical trials to ensure its safety and effectiveness. In one trial, patients who received the WT1 vaccine had a median overall survival of 21.4 months, compared to 16.6 months for those who received standard therapy. Doctors are also testing vaccines with immunotherapy drugs such as nivolumab (Opdivo).
Virotherapy
Virotherapy has existed since the 1950s but has recently become a new treatment for mesothelioma. There are two types of virotherapy: Viral immunotherapy uses viruses to help your immune system attack cancer cells, and oncolytic viruses kill the cancer cells directly.
Viral vectors are modified viruses that deliver immunotherapy drugs or gene therapy into tumor cells. The viral activity also triggers the immune system to respond and attack the cancer site. Some virotherapy studies for mesothelioma have demonstrated improved disease response and overall survival.
Multimodal Therapy: Combining Mesothelioma Treatments
Multimodal therapy is the term for combining multiple treatments, such as mesothelioma surgery and emerging therapies. Doctors can use numerous techniques together to control tumor growth and cancer spread.
In most cases, this approach is more effective than either treatment alone. For example, postsurgical radiation for mesothelioma can kill cancer cells left behind after surgery. However, patients in a multimodal plan are also subject to side effects from multiple treatments.
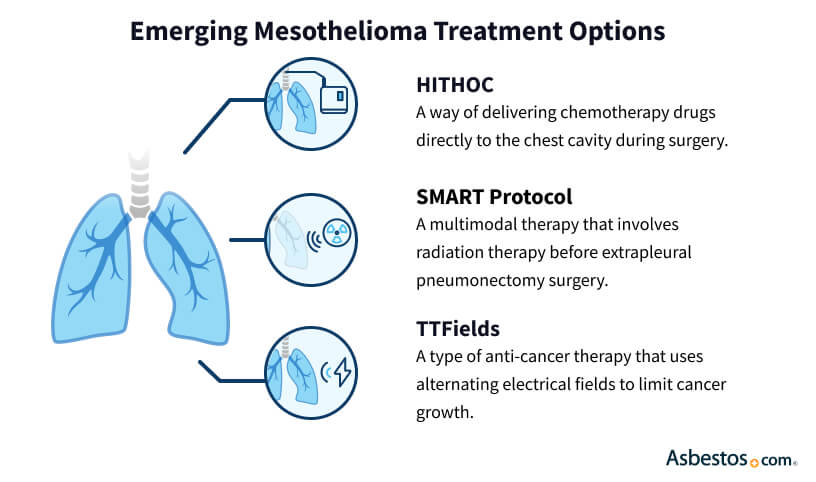
Emerging cancer treatments give physicians more options. Tumor-treating fields helped control tumor growth in clinical trials. The FDA approved TTFields for mesothelioma in 2019, and it’s now part of a standard multimodal plan with chemotherapy.
Hyperthermic intraperitoneal chemotherapy involves circulating heated chemotherapy through the abdomen during surgery. A similar technique kills cancer cells throughout the thoracic cavity. Another emerging multimodal treatment option is surgery for mesothelioma after radiation. In this SMART protocol, patients undergo radiation therapy before extrapleural pneumonectomy surgery.
How to Access New Mesothelioma Treatment
Clinical research trials allow people to access new and emerging mesothelioma treatment options. These trials are often limited to specialized treatment centers. Patients must also pass an eligibility checklist to ensure their safety.
One of the most common questions patients ask us is what clinical trials of new mesothelioma treatments are available. We can help guide them to these types of resources.
Examples of eligibility requirements for a clinical trial include cancer stage, cell type, tumor location, overall health and previous treatment history. Patient Advocates at The Mesothelioma Center can help patients interested in emerging therapies find eligible clinical trials and treatment centers in their area.
John Fiala joined a unique clinical trial using the immunotherapy combination of Opdivo (nivolumab) and Cyramza (ramucirumab). The treatment has enabled his immune system to fight the cancer in two different ways. Opdivo blocks a protein that often prevents the immune system from attacking tumors. Cyramza decreases the blood supply tumors need to metastasize.
Participating in a Clinical Trials
Speaking with your mesothelioma specialist is one of the first steps in participating in a clinical trial. If you’re unsure how to enroll in clinical trials in your area, The Mesothelioma Center Patient Advocate team can match you with local studies.
Many patients benefit from a clinical trial when other options have failed. In a research study, you’ll have access to the newest medications. Study sponsors often cover the entire cost of the experimental treatment. If you wish to drop out or aren’t receiving a benefit at any point during the study, your doctor will switch you to a standard-of-care treatment.
Mesothelioma Specialists
Specialists offer the newest treatment options available. They have the knowledge and experience to diagnose asbestos-related illnesses quickly and accurately. They also know the best multimodal treatment plans for each patient’s diagnosis.
Finding a mesothelioma specialist can be challenging. Getting a second opinion or referral can make a significant difference. Specialists are involved in the latest mesothelioma research using cutting-edge technology.
Mesothelioma Cancer Centers
Emerging mesothelioma therapies and clinical trials are available at specialized cancer centers. These facilities offer new mesothelioma treatment options.
The teams at top mesothelioma centers include world-renowned oncologists, surgeons and other experts. At most centers, these experts meet to deliberate the best mesothelioma treatment options for each patient.

How Does the FDA Approve New Mesothelioma Treatments?
The U.S. Food and Drug Administration’s approval process involves several checks to ensure the safety and effectiveness of new treatments. Researchers prove safety during the discovery phase and in preclinical research.
Human participants then join the clinical testing phase. The FDA reviews trials and conducts postmarket monitoring for safety.
- Preliminary lab tests
- Investigational New Drug application
- Clinical research trials
- FDA review of trial research and scientific data
- FDA inspection of manufacturing facility
Pharmaceutical companies must prove their drug’s benefits outweigh its risks. The FDA has a five-step approval process for new drugs, and its Center for Drug Evaluation and Research sets criteria for drug quality, safety and effectiveness.
The CDER oversees these standards for prescription and over-the-counter drugs. Other FDA centers test the safety of medical devices, food, veterinary medicines and tobacco products.
Common Questions About Emerging Mesothelioma Treatments
- How do emerging mesothelioma treatments differ from standard treatments?
-
Emerging mesothelioma treatments are under scientific investigations, unlike standardized and traditional therapies. However, emerging therapies offer potential patient benefits through new technologies and medical applications that could help those whose disease has yet to respond to other options.
- Am I a candidate for new mesothelioma treatments?
-
Eligibility for new mesothelioma treatments depends on cancer stage, type, overall health and more. You’ll need to weigh the risks versus benefits of any new treatment or clinical trial. Your mesothelioma specialist or a Patient Advocate can help you determine which clinical trials for emerging and new treatments could benefit you most.
- Are there financial assistance options available to help me pay for emerging mesothelioma treatments?
-
Clinical trial sponsors cover the cost of emerging and new mesothelioma treatments during research trials. Travel grants, reimbursements, waivers and other financial assistance are available for clinical trials and new therapies.








