More Mesothelioma Cancer Centers Using Tumor Treating Fields
Treatment & DoctorsWritten by Sean Marchese, MS, RN | Edited By Walter Pacheco
Tumor Treating Fields is one of the newest treatments on the market for pleural mesothelioma cancer patients with metastatic disease. The device is also available to patients with locally advanced cancer who are not candidates for mesothelioma surgery.
Clinical trials of the treatment, previously known as NovoTTF-100L, extended the survival of mesothelioma patients by more than six months compared to those receiving only chemotherapy.
The device is now called Optune Lua and has been available outside of clinical trials for over a year. More physicians and treatment centers are becoming certified to prescribe the treatment alongside pemetrexed (Alimta) and platinum-based chemotherapy.
Over 100 Physicians Now Prescribing Optune Lua
Novocure, the device’s manufacturer, recently announced that more than 100 physicians from over 50 cancer treatment centers within the U.S. are certified to prescribe Optune Lua.
At least 28 of the 50 certified centers are actively offering Optune Lua to malignant pleural mesothelioma patients. Additional centers are working toward completing all certification requirements.
Notable centers currently prescribing Optune Lua include:
New mesothelioma treatments options provide hope to patients with this rare disease, explained Dr. Matthew T. Ballo, professor and chair, Department of Radiation Oncology, West Cancer Center & Research Institute in Tennessee.
“This is a disease with a poor prognosis, so being able to offer patients a therapy that results in excellent response rates with minimal toxicity is exciting,” Ballo said. “We, as clinicians, can have a big impact on our patients’ lives by making them aware of this important new technology.”
Mesothelioma doctors have been eager to prescribe the treatment for patients who have had historically few therapy options.
“I think it is a good treatment in the context of the lack of options for patients with metastatic or unresectable tumors,” said Dr. Alan Dal Pra, radiation oncologist at the University of Miami’s Sylvester Comprehensive Cancer Center.
Pritesh Shah, Novocure’s chief commercial officer, is excited about the increased availability of this compelling new treatment.
“We are proud of the progress we have made in the last year in making Optune Lua accessible to patients,” Shah said. “We continue to work diligently to expand the number of centers that can provide Tumor Treating Fields therapy to patients facing this devastating and aggressive disease.”
Access top mesothelioma cancer centers that have experience treating this rare disease.
Get Help NowAlternating Electrical Fields Disrupt Mesothelioma Tumor Growth
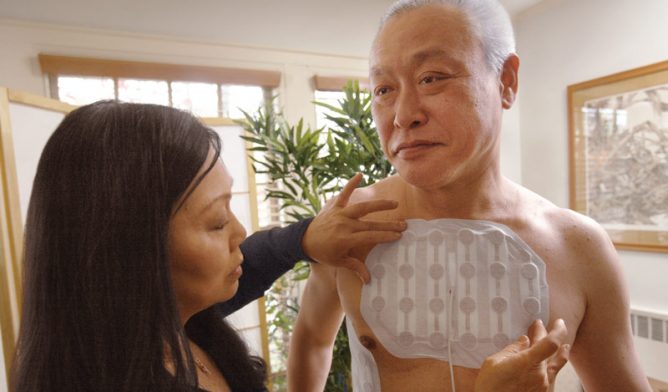
Tumor Treating Fields works by delivering electrical frequencies through adhesive pads that are attached to the chest. The pads connect to a personal device that produces painless electrical fields that interrupt cancer cell division and stop tumors from growing.
The electric fields can also cause affected cancer cells to die. TTFields does not heat or stimulate the skin and causes minimal damage to healthy cells. It is one of few therapies showing promise for aggressive solid tumor cancers, such as mesothelioma.
Novocure has been developing the technology since 2000, and the FDA has approved the treatment for pleural mesothelioma, brain cancer and lung cancer.
Patients can carry the personal device in a carrying bag or backpack, allowing them to perform daily activities while receiving treatment. Patients report that the most common side effect is mild skin irritation. Novocure advises against the use of TTFields for patients with an implanted medical device, such as a pacemaker.
Physicians prescribe chemotherapy for mesothelioma patients every three weeks while they are on TTFields. Patients receive Alimta with either carboplatin or cisplatin for a total of six cycles.
During mesothelioma clinical trials for the device, 97% of patients reported tumor shrinkage or no new growth. The average survival for epithelial mesothelioma patients was 21.2 months, and participants in the trial had no new cancer growth for an average of 7.6 months.
Dr. R. Taylor Ripley, director of the Mesothelioma Treatment Center at Baylor College of Medicine, sees TTFields as a new opportunity for patients.
“We are always looking for treatments to improve the survival, quality of life and options for patients,” Ripley said. “This provides another therapeutic option that may be valuable.”