Immunotherapy for Mesothelioma
Mesothelioma immunotherapy uses drugs to boost the patient's immune system to find and fight cancer cells. It improves the body's response against mesothelioma. Benefits include longer life, better quality of life and fewer symptoms.
What Is Immunotherapy for Mesothelioma?
Immunotherapy for mesothelioma is a treatment that uses the immune system against asbestos-related cancer. It’s now a standard treatment for many patients. Immunotherapy boosts the immune response, while chemotherapy and radiation target cancer cells. This method offers excellent hope for mesothelioma patients with difficult-to-treat forms.
Key types of immunotherapy include checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo). These drugs block proteins that delay immune attacks against cancer. Monoclonal antibodies also target cancer cells. Research shows these treatments improve survival with fewer side effects. Doctors often combine them with chemotherapy or surgery.
- It’s among the top treatments for mesothelioma.
- About 17% of patients in remission used it.
- Some trials extended survival by over 1 year.
- Chemoimmunotherapy often outperforms chemotherapy alone.
Peritoneal mesothelioma survivor Tami Pream used immunotherapy and HIPEC to keep her cancer from returning. She said her treatments are going well so far, and she’s not worried about any side effects.
“Immunotherapy has revolutionized survival for patients with cancers that were historically very difficult to treat like mesothelioma,” said Dr. Andrea Wolf, director of the New York Mesothelioma Program at Mount Sinai.
How Does Immunotherapy for Mesothelioma Work for You?
Immunotherapy for mesothelioma works by kickstarting the immune system’s ability to find and kill cancer cells. It’s like when your body fights viruses and bacteria. Immune cells also clear out damaged cells.
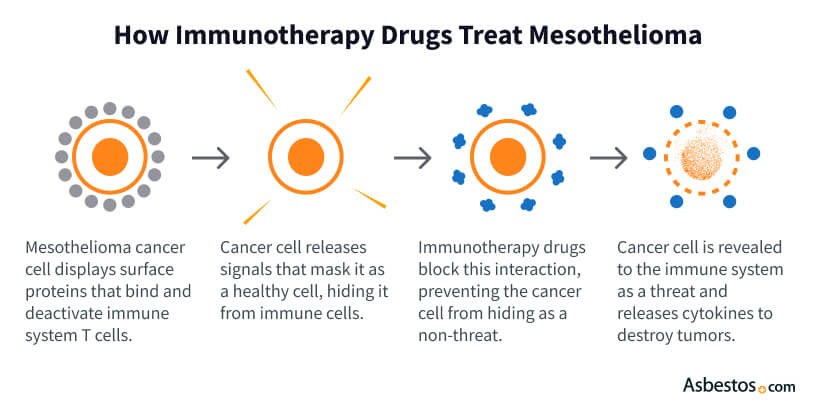
Cancer cells can hide from the immune system by using specific proteins. Immunotherapy drugs counteract this. They expose cancer cells, allowing immune cells to attack.
Checkpoint inhibitors like Keytruda and Opdivo expose tumors to the immune system. This helps immune cells destroy them.
What Are the Types of Immunotherapy for Mesothelioma?
There are several types of immunotherapy used to treat mesothelioma. Each targets the immune system differently. The main types are cancer vaccines, monoclonal antibodies, immune checkpoint inhibitors and CAR T-cell therapy.
Immunotherapy can be active or passive. Active types, like CAR T-cell therapy, stimulate antibody production. Passive types, like Keytruda and Opdivo, use engineered immune cells for protection.
Clinical trials test new immunotherapy types. Success varies by patient. Factors include cancer type, stage, past treatments, and health.
Mesothelioma Cancer Vaccines
There are preventive and therapeutic vaccines. Preventive vaccines lower the chance of recurrence. Therapeutic vaccines aim to treat active diseases. It’s important to remember that vaccines are not a cure for mesothelioma.
Research is ongoing for a preventive vaccine targeting the OX40 receptor. It has shown promising results in mice. Two therapeutic vaccines, CRS-207 and galinpepimut-S (WT1), are in trials. Both have shown improved survival.

Cell and Protein Therapies
Cell therapies involve injecting immune cells into patients. These are promising for other cancers and are being tested for mesothelioma. CAR T-cell therapy modifies T cells to target cancer.
In one trial, patients received dendritic cells after chemotherapy. These patients showed strong immune responses. Cytokines are proteins that enhance immune responses. These proteins can kill cancer cells or stop their growth.
Monoclonal Antibodies
Monoclonal antibodies create copies of anti-cancer antibodies. They’re common in cancer treatment but rare for mesothelioma. Research is ongoing to change this.
Tremelimumab is one MAB under investigation. It targets CTLA-4. Another, amatuximab (MORAb-009), shows promising results in trials.
How Effective Is Immunotherapy for Mesothelioma?
The success rate of immunotherapy varies by patient’s health and cancer stage. Key drugs include Opdivo, Yervoy and Keytruda. Trials show promising results, especially for recurrent cancer.
JAMA Network Open in 2024 published a study on the success of immunotherapy. Researchers said their results “support pembrolizumab use as a treatment option” for mesothelioma patients.
- Keytruda offers 18 months of survival for recurrent cases.
- Opdivo and Yervoy provide 18.1 months on average.
- Immunotherapy patients have a 41% two-year survival rate, compared to 27% with chemotherapy.
The FDA has approved Opdivo and Yervoy for certain patients. New treatments, including immune checkpoint inhibitors and the WT1 vaccine, are being tested.
What Are the Best Immunotherapy Treatments for Mesothelioma?
Combining Opdivo and Yervoy boosts survival rates. Some trials show a two-year survival rate of about 40% for advanced mesothelioma. Keytruda, used after first-line treatment, shows a 22% response rate. Some patients benefit long term.
Ongoing trials aim to better understand these treatments. Not everyone responds, but some enjoy longer lives with stable disease. Patient stories highlight immunotherapy’s potential.
Opdivo and Yervoy
Opdivo and Yervoy target different immune system checkpoints. Opdivo blocks PD-1, helping the immune system find cancer. Yervoy blocks CTLA-4, enhancing the immune response. Together, they improve the body’s fight against mesothelioma.
Emily Ward, a survivor, showcases the success of this treatment. After other treatments failed, she combined Opdivo and Yervoy. Her tumors shrank, and her life improved. Emily’s story shows that this combo can extend survival.
Keytruda
Keytruda is for patients whose cancer worsens after initial treatments. It blocks the PD-1 pathway, aiding the immune system. It is a second-line treatment when cancer recurs or advances.
Keytruda shows promise with a 22% response rate. It extends life and improves the quality of life when other treatments fail. Mesothelioma survivor Barbara Lapalla said, “Keytruda has worked like a charm for me, although I’m not sure why.” There have been no side effects. For me, it’s the magic bullet.”
Common Side Effects of Immunotherapy for Mesothelioma Patients
Immunotherapy can boost side effects because of an increased immune response. Common issues include fevers and body aches. Coughing and fatigue are especially bothersome.
- Constipation
- Coughing
- Diarrhea
- Fatigue
- Fever
- Loss of appetite
- Mouth sores
- Muscle or joint pain
- Nausea
- Skin irritation
- Weight loss
Most patients experience mild, temporary side effects. Severe reactions are rare but can harm tissues or organs. These are often due to inflammation.
- Pneumonia: Chest pain, shortness of breath and persistent cough
- Colitis: Inflammation of the colon that causes bloody stools, abdominal pain and intestinal tearing
- Hepatitis: Liver inflammation characterized by eye and skin discoloration, changes in urine and right-side abdominal pain
- Hormone Gland Problems: Hormonal side effects such as muscle aches, increased heart rate, headaches and weight loss
- Kidney Problems: Nephritis, or inflammation of the kidneys, leading to changes in urine, back pain, hormone imbalance and potential kidney failure
Early treatment of side effects is key. Discuss benefits and risks with a specialist. Lifestyle changes, like a specific diet, can help.

Benefits of Immunotherapy for Mesothelioma
Immunotherapy offers longer survival and better symptom control for mesothelioma. It targets cancer cells without harming healthy tissue, unlike chemotherapy or radiation.
- Fewer side effects and more manageable than conventional mesothelioma treatment.
- Financial assistance is often available through clinical trials or grants.
- Personalized treatment for each patient based on their DNA.
- Targeted immunotherapy reduces damage to healthy tissues.
- Treatment uses the immune system and the body’s cells to fight cancer.
- When a patient enrolls in a clinical trial, the research sponsor covers the cost of experimental immunotherapy.
Immunotherapy and similar treatments are the future for mesothelioma. They promise lasting protection against the disease.
40%
Percentage of respondents who tried immunotherapy in The Mesothelioma Center’s patient survey.
Source: State of Mesothelioma
Cost of Immunotherapy
Immunotherapy for mesothelioma can cost over $10,000 a month. Adding more care raises this cost. The combo of Yervoy and Opdivo surpasses $250,000 a year. Other treatments can hit $150,000.
Medicare and many insurances cover it. However, patients might face out-of-pocket costs and lost wages. VA benefits lower costs significantly, but the claims process is tricky.
Patient Advocates can help find financial aid. The Mesothelioma Center’s Veterans Outreach team assists with VA claims.
We do our best to explain patients’ financial options. Our job as Patient Advocates is to educate and guide them, so when it comes time to make those decisions, they’re making very informed decisions.
What Is the Future of Immunotherapy for Mesothelioma?
Researchers aim to combine immunotherapy with other treatments. In May 2024, the FDA fast-tracked Keytruda’s review with chemotherapy for pleural mesothelioma.
Other clinical trials are testing several immunotherapy and chemo combinations for pleural and peritoneal mesothelioma. These trials stem from the promising results of previous experiments. The novel therapy combination shows hope for inoperable mesothelioma patients.
- Some types of immunotherapy for mesothelioma have already been approved for advanced, malignant and inoperable cases, according to a 2025 study published in the medical journal Cancers. Clinical trials are exploring more immunotherapy and chemotherapy combinations as first-line treatments.
- Our exclusive 2023 survey found that 40% of mesothelioma patients underwent immunotherapy as part of their personalized treatment plan.
“This has really helped improve treatment outcomes in mesothelioma, whether in unresectable or even respectable mesothelioma; thus, more research in immunotherapy is crucial to improving outcomes.”
Common Questions About Immunotherapy for Mesothelioma
- What is the success rate of immunotherapy?
-
Success varies by patient, cancer type, stage, and immune system. Keytruda is a top choice for malignant pleural mesothelioma. In the KEYNOTE-483 trial, it cut death risk by 21% when combined with chemotherapy. Keytruda often adds nearly a year to life expectancy.
- Is immunotherapy equally effective for men and women?
-
A 2018 study found men had a 28% lower death risk on checkpoint inhibitors. Women had a 14% lower risk. Both genders benefit from immunotherapy, with no significant response differences.
- What are the disadvantages of immunotherapy?
-
Outcomes depend on the patient’s immune system and tumor markers. Side effects and symptoms can worsen based on immune response intensity.
- Is immunotherapy better than chemotherapy?
-
Both treatments have pros and cons. Immunotherapy may cause fewer side effects. However, chemotherapy might control tumor growth better. Many doctors recommend combining both for the best results.






